Samples & Data Access and Analysis
*** A complete dataset for CALERIETM to July 1, 2020 is available via this CALERIETM Research Network site. However, a more updated database after July 1 2020 is available from the NIA site.***
This section includes all the information needed to access and use the CALERIETM Research Network resources, including the public use database and the application for access to biological samples.
Help us help you! Although access to the study database is open, the CALERIETM External Science Committee requests completion of this form so that we can better assist you when you seek guidance on use of the dataset.
Before proceeding with a study project, please check the Ongoing Projects tab to see studies currently in progress.
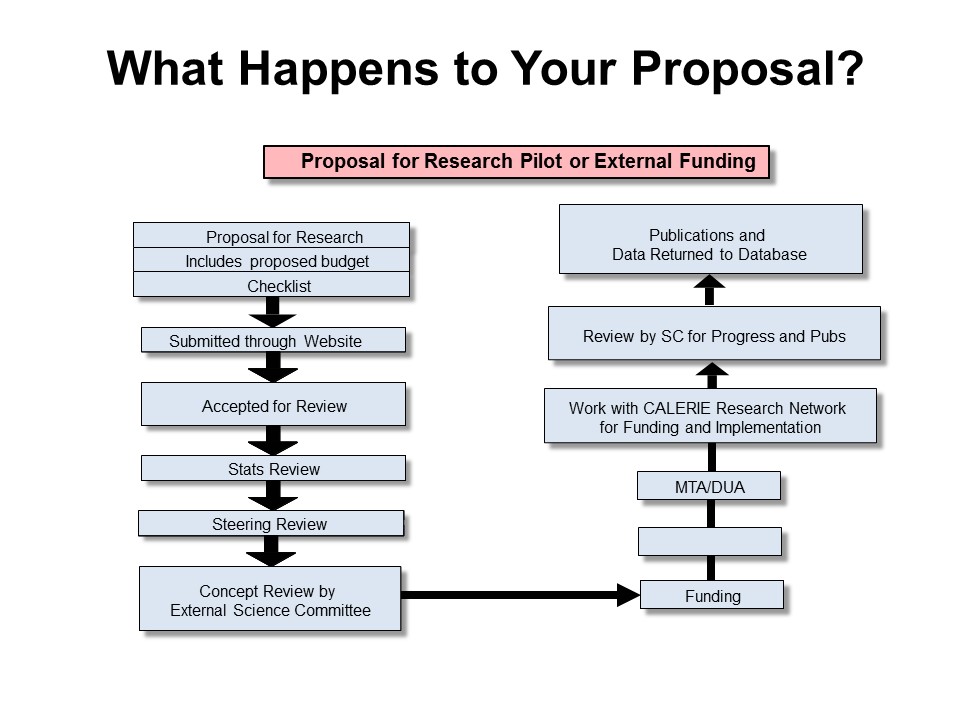
All proposed ancillary studies and secondary data analyses must be submitted to the SC in time for subsequent SC review prior to submission to a funding agency. The Committee meets monthly for regular business and consideration of ancillary studies. Studies submitted for review less than 60 days prior to a funding application submission deadline may not receive an approval letter for inclusion in the application. Further, grant applications submitted for funding not receiving approval in advance for use of samples cannot be guaranteed approval for access post hoc, even if funding is obtained.
We are deeply grateful to the individuals who volunteered for participation in the study. Without their dedication and involvement, this research would not be possible.
- Guide to using the Public Use Database (read before doing analyses) - helpful information for the Network Investigator
- CALERIETM Sample Access and Ancillary Study Guidelines - the operating principles and procedures applicants are asked to follow to obtain access to samples
- CALERIETM Sample Repository - overview of the types of biological samples available.
Note: some baseline, 12-month; and 24-month arterialized plasma samples were collected from a hand vein using a hot-box chamber. These samples were used only for determination of plasma catecholamine concentrations and were not used for any other purpose. None of these samples remain in the biorepository. All other samples used for other analyses and for future use were collected under standard conditions. - Click here to submit your Ancillary Study Application.
- Click on the image below to view an enlarged diagram of the proposal process